IUCLID format
From this page you can download information related to the IUCLID 6 format for the major versions of IUCLID 6 that have been published to date. The archives per major version are:
- IUCLID 6 v8, published on 29th of April 2024
 (.zip | 37.1 MB)
(.zip | 37.1 MB) - IUCLID 6 v7, published on 19th of May 2023
 (.zip | 31.8 MB)
(.zip | 31.8 MB) - IUCLID 6 v6, published on 17th of October 2021
 (.zip | 25.1 MB) - (updated on 16.12.2021)
(.zip | 25.1 MB) - (updated on 16.12.2021) - IUCLID 6 v5, published on 28th of October 2020
 (.zip | 24.7 MB)
(.zip | 24.7 MB) - IUCLID 6 v4, published on 30th of October 2019
 (.zip | 20.2 MB)
(.zip | 20.2 MB) - IUCLID 6 v3, published on 24th of October 2018
 (.zip | 16.1 MB)
(.zip | 16.1 MB) - IUCLID 6 v2, published on 15th of November 2017
 (.zip | 15.0 MB)
(.zip | 15.0 MB) - IUCLID 6 v1, published on 29th of April 2016
 (.zip | 14.7 MB)
(.zip | 14.7 MB)
The format is expressed for all IUCLID 6 entities and documents in the general-purpose mark-up language XML, and XML Schema definition files (.xsd). In addition, the format is made available in a more readable version (.doc).
IUCLID 6 is built as a platform and is a modular system. The IUCLID 6 format is divided up in to different groups ('definition providers') depending on its source identified in the section below. To allow data to be exchanged, it can be exported and imported in the IUCLID i6z format. The following document explains the structure of IUCLID i6z files:
- Developers' Guide to the IUCLID i6z Format (.pdf | 0.8 MB) - updated on 18/07/2024
More information on how the IUCLID format is used in specific contexts can be found hereafter:
- EU Poison Centres Notification format
- EU Waste Framework Directive: SCIP database format
- EU Classification and Labelling regulation notification format
- EU Biocidal Product Regulation: Summary of Product Characteristics format
Update of the IUCLID format
The IUCLID format is maintained and updated by the IUCLID team in collaboration with the users community, once a year. The format update process follows the following timeline:
- Continuous activity: collection of change requests
- September / October: review of the changes and impact assessment by all IUCLID users and relevant OECD groups. Draft changes are shared with users, on this page
- April: publication of the IUCLID release containing format changes
To share format change requests, please contact either:
- A specific IUCLID user group
- The ECHA Helpdesk or the IUCLID 6 functional mailbox: iuclid6(at)echa.europa.eu
IUCLID 6 definition providers and format
The IUCLID format is composed of different definition providers which are grouping the elements of the IUCLID format according to the level of harmonisation and the specific groups of users responsible for the management of the format. You will find more information on the IUCLID users on the IUCLID Product page. The existing definition providers are:
- Harmonised at the OECD level
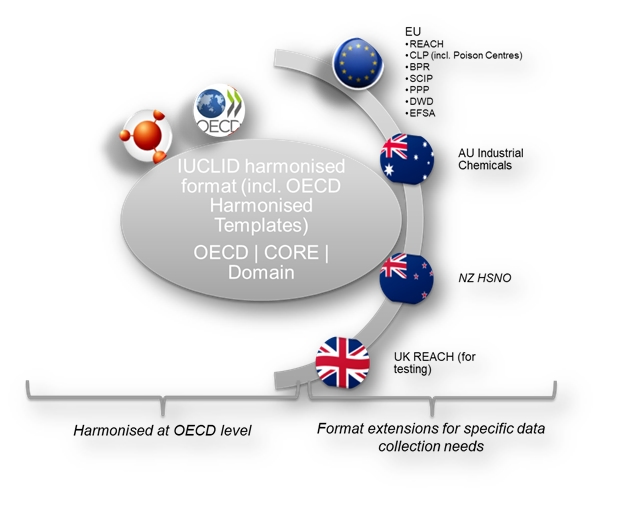
- OECD contains the set of documents harmonised at the OECD level to report studies done on chemicals to determine their properties or effects on human health and the environment.
- CORE (IUCLID) contains the main documents of IUCLID such as the substance identification, the classification and labelling information or the endpoint summaries.
- DOMAIN, introduced in IUCLID 6 v3, contains the main entities of the IUCLID application. It is a mandatory module containing all entities used in IUCLID.
- Specific definition providers
- AU_IND_CHEM, introduced in IUCLID 6 v3, contains all documents and rules that are required by a company to create and manage submissions that are relevant to the Industrial Chemicals Law administered by the Australian Government's Department of Health.
- EU BPR contains the specific documents used for the european Union Biocidal Products Regulation - BPR.
- EU CLP contains the specific documents used for the european Union CLP Regulation. It also contains the Poison Centres Notification (PCN) format specifications. For more information on the PCN format, please refer to the Poison Centres website.
- EU PPP, introduced in IUCLID 6 v4, contains the configuration of the Plant Protection Products and active substances dossiers to be used in a pilot study performed in collaboration with EFSA.
- EU REACH contains the specific documents used for the European Union REACH Regulation, such as the inquiry or the opt-out information.
- EU SCIP, introduced in IUCLID 6 v4, contains the specific documents and configuration for the needs of the database which will contain the submitted information on Substances of Concern In articles, as such or in complex objects (Products) - SCIP. More information is available here.
- NZ HSNO, introduced in IUCLID 6 v4, contains the specific format and configuration to be used in the future hazardous substance database of the New Zealand EPA.
- EU EFSA, introduced in IUCLID 6 v7, contains the format supporting the publication of relevant databases by EFSA.
- EU ECHA, introduced in IUCLID 6 v7, contains the format supporting the publication of relevant databases by ECHA.
- UK REACH, introduced in IUCLID 6 v7, contains a set of configuration that will be used for testing customisation possibilities for UK. The content will be managed by HSE. This is not in production use.
- EU DWD, introduced in IUCLID 6 v7, contains the draft format for supporting the information requirements collection under the EU Drinking Water Directive.
The following information is also available in the above format information packages:
- IUCLID 6 document schemas (.xsd files), including for additional IUCLID 6 files elements (e.g. platform fields, manifest file structure).
- IUCLID 6 list of all fields contains the unique IUCLID field identifers. These can be accessed in the IUCLID user interface, by expanding the information icon next to each field. This file also contains the IUCLID 6 phrases and phrase groups displayed as picklists in IUCLID.
- Each format information package contains an Excel file, per definition provider (e.g. CORE, OECD, EU BPR), detailling all the changes made between two consecutive IUCLID versions (e.g. between IUCLID 6.2 and IUCLID 6.1).
- Since IUCLID 6.4, the following additional information is included in the format definition package above:
- Migration rules, including phrase group mapping, from the previous version of IUCLID to the new one
- Dynamic content rules, to describe the conditional display rules linked to specific IUCLID fields in the IUCLID 6 web interface
Background information on the IUCLID format
The management of the IUCLID format is done by the European Chemicals Agency (ECHA) in collaboration with the OECD. ECHA's mandate originates from REACH Article 111 ("Formats and software for submission of information to the Agency"):
"The Agency shall specify formats and make them available free of charge [...] on its website for any submissions to the Agency. Member States, manufactures, importers, distributors or downstream users shall use these formats [...] in their submissions to the Agency pursuant to this Regulation. [...] For the purposes of registration, the format of the technical dossier [...] shall be IUCLID. The Agency shall coordinate the further development of this format with the Organisation for Economic Cooperation and Development to ensure maximum harmonisation."
The IUCLID 6 application follows this format intrinsically and IUCLID 6 users are, by definition, compliant with the format requirement of the REACH regulation and also compatible with the OECD Harmonised format.

