REACH and CLP
| Report Template | Output(s) | Preview | Report description |
|---|---|---|---|
|
REACH Chemical Safety Report (CSR) Latest version with version 7.12.4 of IUCLID 6 |
  
|
 |
The chemical safety report documents the chemical safety assessment undertaken as part of the REACH registration process, and is the key source from which the registrant provides information to all users of chemicals through the exposure scenarios. It also forms a basis for other REACH processes including substance evaluation, authorisation and restriction. |
| Assessment entity report for nano forms |
|
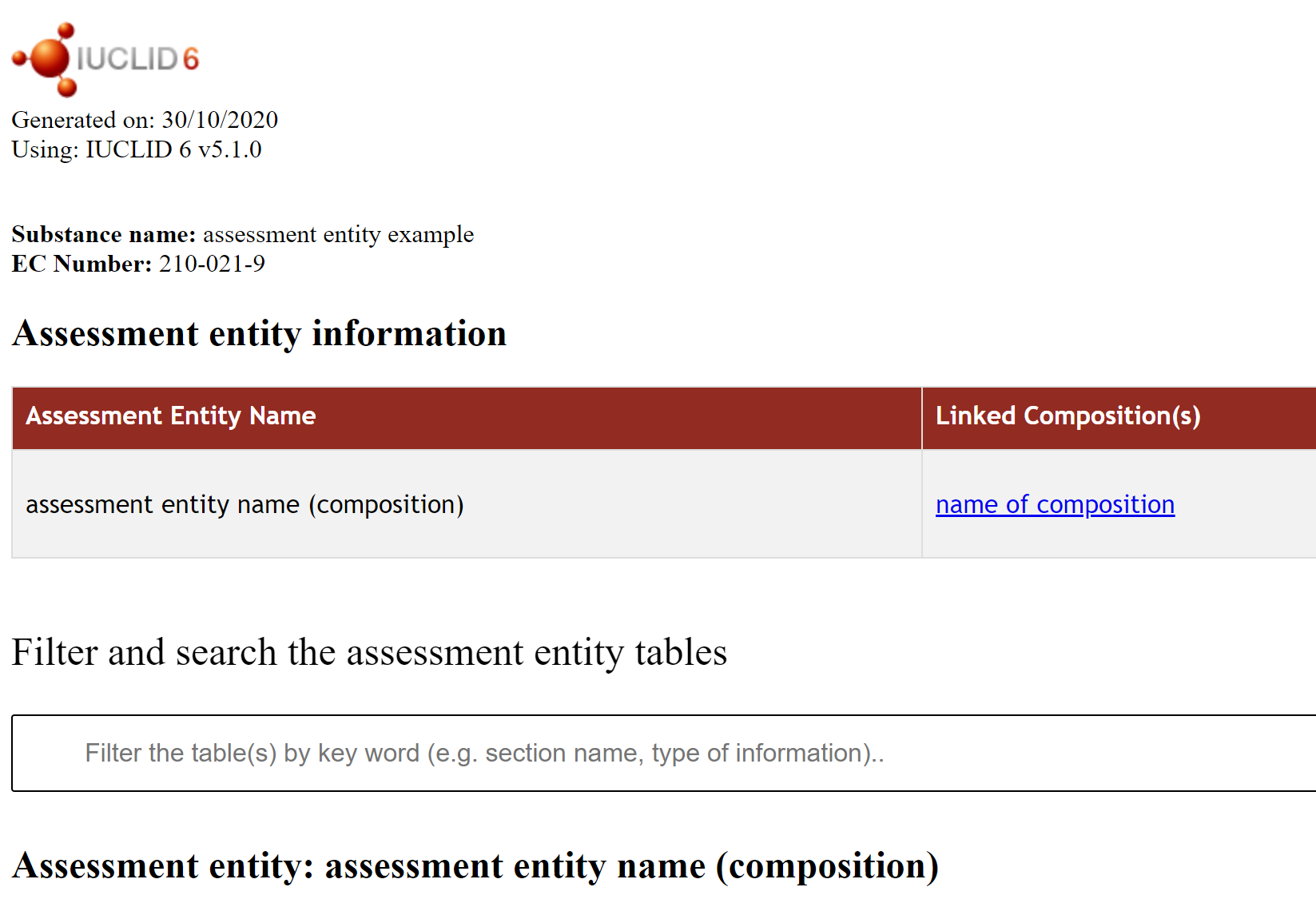 |
Under the amendment to the REACH regulation (2018/1881) of 3 December 2018 to address nanoforms of substances, nanoforms of a substance need to be covered by the registration dossier. Typically, nanoform dossiers are prepared using the IUCLID assessment entity approach (see the guidance). This report shows in the first table the referenced composition(s) and endpoint summaries inside each assessment entity (section 1.10 – ‘Specific composition / form of the registered substance’). There is a new row per assessment entity document. The report shows in the second table the referenced endpoint summary information inside a single assessment entity, as well as information from the endpoint studies which are referenced by those endpoint summaries. There should be one table generated per assessment entity document, with a new row per referenced endpoint summary. |
| Substances of very high concern report (REACH Annex XV) |
|
 |
Member States or the European Chemicals Agency (ECHA), on request of the European Commission, may propose a substance to be identified as an SVHC by preparing a dossier in accordance with the requirements set out in Annex XV to REACH. This report is a draft version of the SVHC report submitted with the proposed substance. |
| Composition of a substance |
|
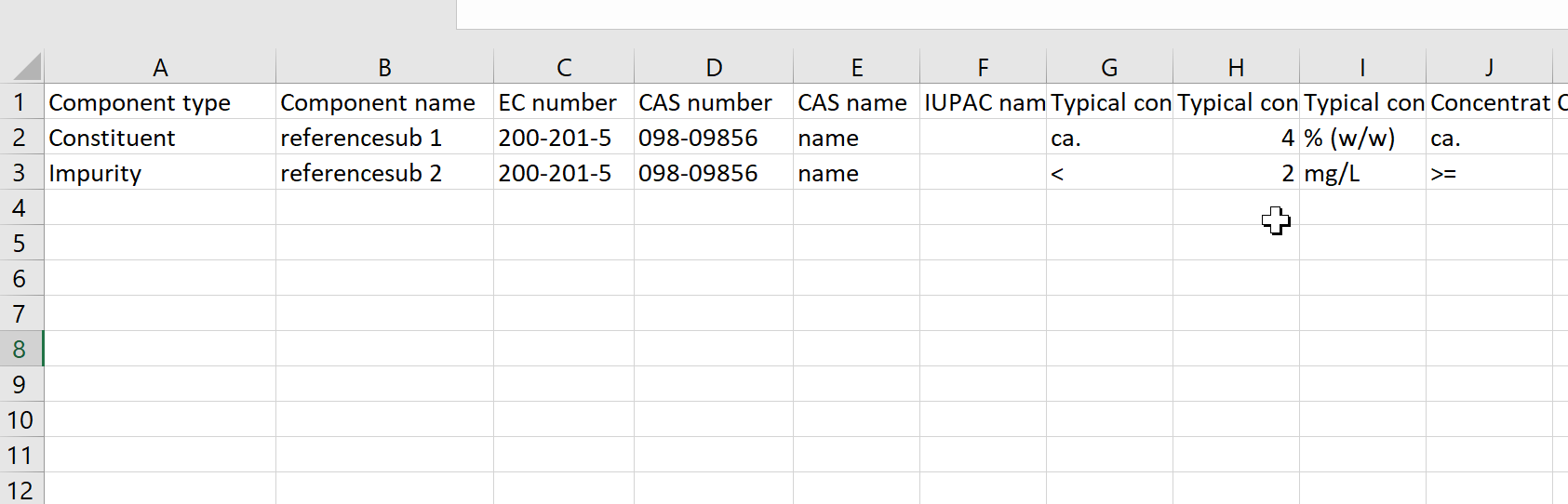 |
This report outlines the basic substance composition details. The units and qualifiers of the constituents, impurities and additives are spearated into different columns. The report is mainly used by the 'B1 Chemistry' unit of the European Chemicals Agency (ECHA). |
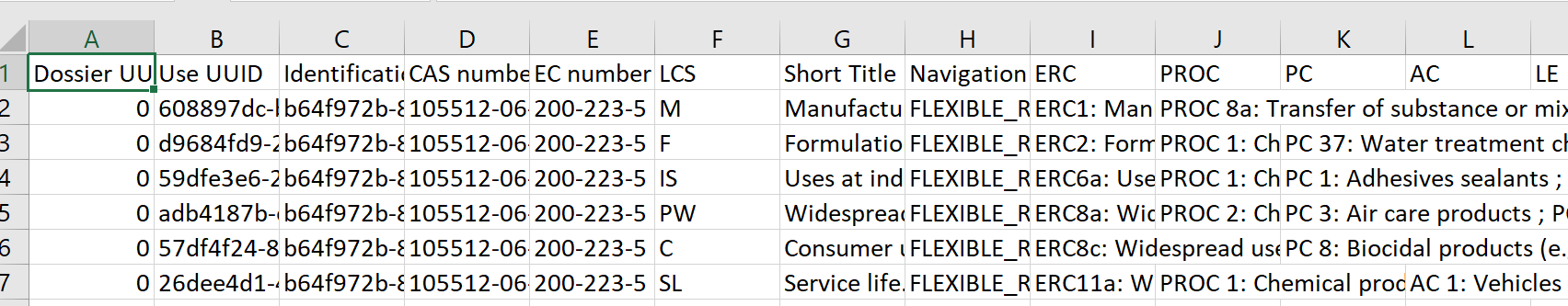
| List of use information (created for and by DOW Chemicals) [compatibility with latest format not guaranteed] |
|
This report lists key information from the Use section (3.5 of REACH) for each use life cycle. Note that the report is now updated to version 6.6 |